
Students should also explain any discrepancies between their results and the publish information.
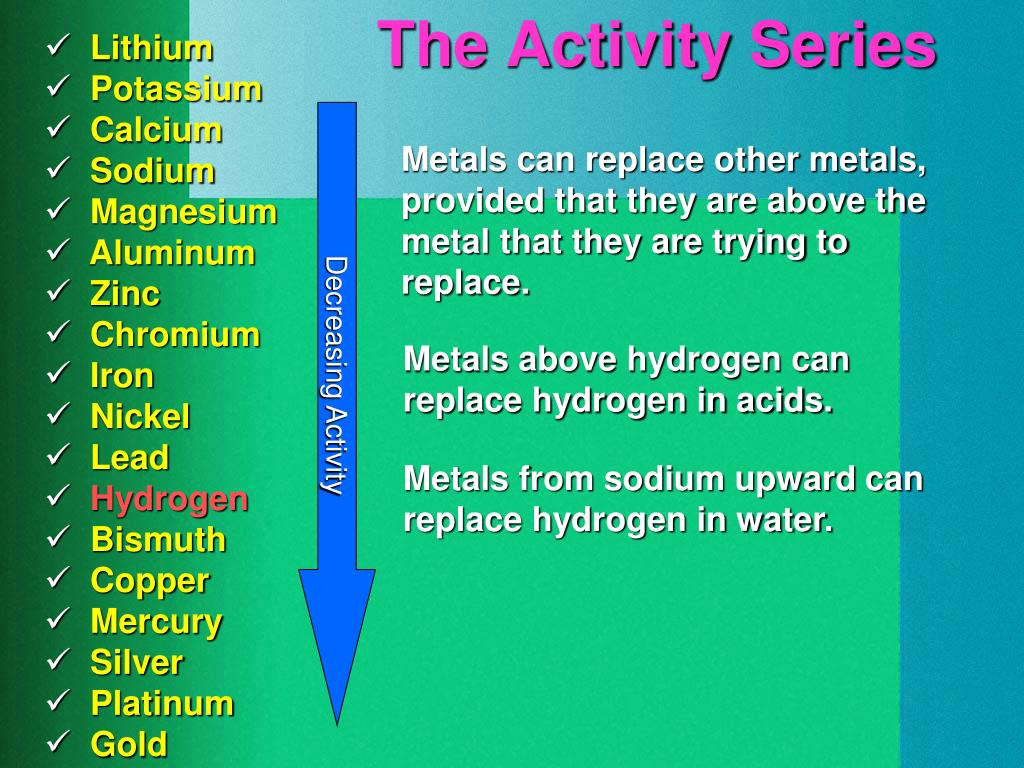
#Chemical activity series series#
Also, the published version of the activity series may be compared to the results. Once this results have been compiled as a class, discuss how the activity aligns with the metals They should be able to justify their decision. The students will be instructed that they are to determine the activity of each of the metals and rank them according to their results. The materials needed for this lab include 4 metal samples- such as magnesium, iron, copper, zinc, and tin-spot plates/test tubes, 0.1M HCl or 1.0M HCl, and a container for disposal of waste products. This will lead to the objective of this lab to determine the activity of four mystery metals.
As students discuss that reasons for the production of hydrogen, ask whether any metal combined with HCl will produce this reaction.

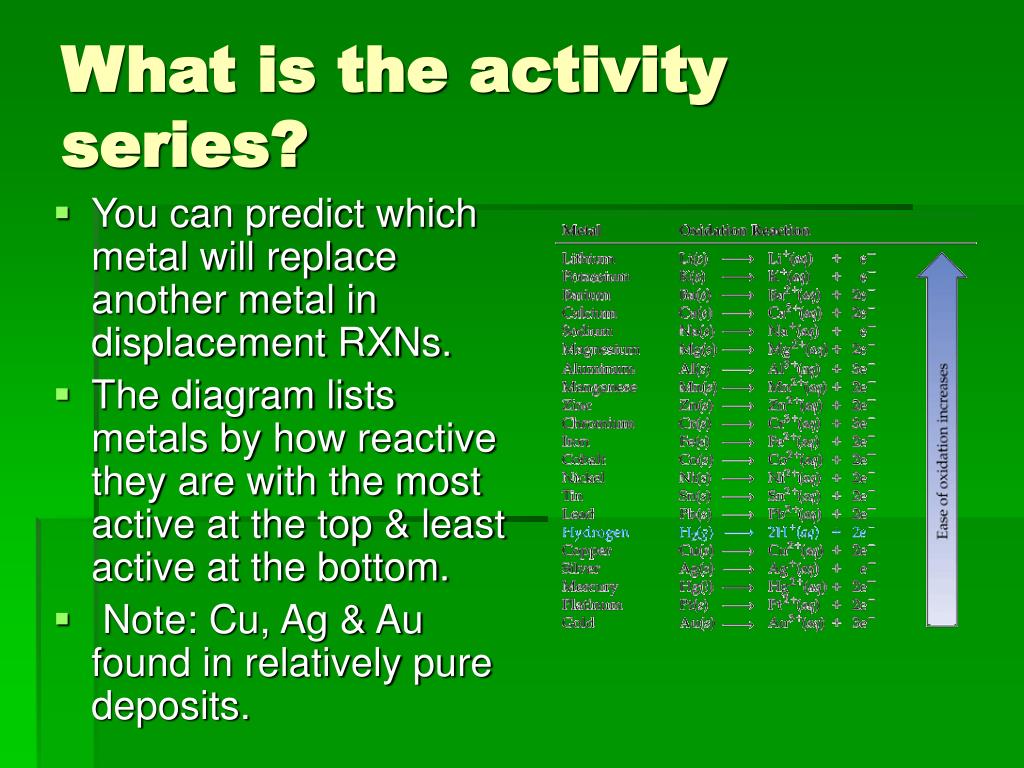
A possible anticipatory set for this activity would be the demonstration of the hydrogen "pop" test. The reactivity series is a series of metals, in order of reactivity from highest to lowest. WARNING: This product can expose you to chemicals including Lead and lead compounds, Potassium Bromate and Strong inorganic acid mists containing sulfuric acid, which are known to the State of California to cause cancer and reproductive harm. The Activity series is a chart which lists metals in order of declining reactivity. For example, Mg is above H in the series. This enables chemists to predict which combinations will undergo single displacement reactions. An element that is higher in the series will displace an element that is below it. It lists the metals and nonmetals in decreasing order of chemical activity. THIS PACKAGE CONFORMS TO 49 CFR 173.4 for domestic highway or rail transport only The Activity Series of metals is a very essential part in predicting the result of many reactions. Chemists have devised an Activity Series. The reactive metal has been oxidized the less reactive metal has been reduced. The more reactive metal will replace the less reactive metal and the less reactive will appear in the solid form. Some might be classified as acid - base, oxidation - reduction, combustion, or as synthesis, double. Associated particle diagrams will be displayed to help students better comprehend the reaction at the particulate level. To empirically determine which of these metals is more reactive, place a piece of the metal in a salt solution of the other. Activity Series is a list of single displacement reactions in order of reactivity. There are many ways to classify chemical reactions. In this simulation, students will reference an activity series and a solubility chart to accurately predict the products of single replacement and double replacement chemical reactions. For example, if we are asked to predict whether Copper or Z. For example if you take the group of metals magnesium, mercury and nickel, magnesium is the most reactive and mercury the least. The Activity Series allows us to predict whether single displacements reactions will take place. This table is similar to the electrochemical series of elements. The relative activity of a species i, denoted a i, is defined as: where i is the (molar) chemical potential of the species i under the conditions of interest, o i is the (molar) chemical potential of that species under some defined set of standard conditions, R is the gas constant, T is the thermodynamic temperature and e is the exponential constant. An activity series of metals is a table of metals arranged in the order of their decreasing chemical activity or the ease at which the metal will give up one or more electrons to form positive ions.


 0 kommentar(er)
0 kommentar(er)
